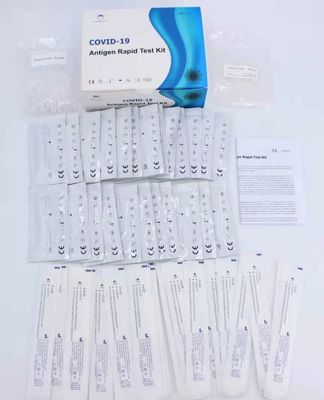
25T/Kit Covid-19 Antigen Test Kit , 0.3kg Nucleic Acid Detection Kit
Product Details:
| Place of Origin: | Beijing |
| Certification: | CE; ISO |
Payment & Shipping Terms:
| Minimum Order Quantity: | 10-100pcs |
|---|---|
| Price: | USD1.8-3.5/pc |
| Packaging Details: | 25pcs per case; box dimension 70.3*51.3*36.3cm |
| Delivery Time: | 3-5working days |
| Supply Ability: | 1million per week |
|
Detail Information |
|||
| Specificity: | 100% | Overall Agreement: | 97.5% |
|---|---|---|---|
| Assay Incubation: | 15minutes, RT | Detection Substance: | Nucleic Acid |
| Format: | 25T/kit | Box Dimension: | 70.3*51.3*36.3cm |
| Highlight: | 25T/Kit Covid-19 Antigen Test Kit,0.3kg Nucleic Acid Detection Kit,25T/Kit Nucleic Acid Detection Kit |
||
Product Description
COVID-19 Antigen Rapid Test Kit Diagnose Throat/ Nasal Fast Test
PRINCIPLEINTENDED USE
COVID-19 Antigen Rapid Test Kit is a solid phase immunochromatographic assay for the in vitro qualitative detection of nucleocapsid protein antigen from 2019 Novel Coronavirus in human nasopharyngeal secretion or oropharyngeal secretion. This test kit provides only a preliminary test result for COVID-19 infection as a clinically-assisted diagnosis.Testing is only limited to professional laboratories .
SUMMARY
The novel coronaviruses belong to the β genus.COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by novel coronavirus are the main source of infection, asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days. The main manifestation include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.Coronaviruses are enveloped RNA viruses that are distributed broadly among humans, other mammals, and birds and that cause respiratory, enteric, hepatic, and neurologic diseases. Seven coronavirus species are known to cause human disease. Four viruses - 229E, OC43, NL63, and HKU1 - are prevalent and typically cause common cold symptoms in immunocompetent individuals. The three other strains - severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and 2019 Novel Coronavirus (COVID-19) - are zoonotic in origin and have been linked to sometimes fatal illness. The COVID-19 Antigen Rapid Test Kit can detect pathogen antigens directly from nasopharyngeal swab,nasal or throat swabs specimens.
INTERPRETATION OF RESULTS
NEGATIVE:
If only the C band is present, the absence of any burgundy color in the T band indicates that no COVID-19 antigens are detected in the specimen. The result is negative.
POSITIVE:
In addition to the presence of C band, if T band also emerged, then the test indicates the presence of COVID-19 antigens in the specimen. The result is positive.
INVALID:
Control line fails to appear. Incorrect procedural techniques and invalid test kit are the most likely reasons for control line failure. Read the instructions carefully again and repeat the test with a new test kit. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
PERFORMANCE CHARACTERCS
1. Clinical Performance of Antigen Test for fresh swabs
Using 127 fresh NP swabs which were collected freshly in Extraction Buffer in the kit to evaluate the Clinical performance of the COVID-19 Antigen Rapid Test Kit. 67 Patients who presented within 7 days of symptom onset and 60 normal persons were included in the initial primary evaluation. The Result is below:
| Sansure Covid-19 RT-PCR Assay | ||||
| Positive | Negative | Total | ||
| Kewei COVID-19 Antigen Rapid Assay | Positive | 64 | 0 | 64 |
| Negative | 3 | 60 | 63 | |
| Total | 67 | 60 | 127 | |
The assay demonstrated acceptable clinical sensitivity for fresh swab samples is 95.52% (95% CI: 87.64%-98.47%) when compared to a molecular device produced by Sansure Inc. The assay demonstrated excellent clinical specificity 100% (95% CI: 93.98%-100%). Overall Agreement was 97.64% (93.28%-99.19%).
2. Overall Clinical Performance of Antigen Test Kit
A total of above 157 positive samples and 260 negative samples were detected to evaluate the clinical performance of the COVID-19 Antigen Rapid Test Kit. The Result is below:
| Sansure Covid-19 RT-PCR Assay | ||||
| Positive | Negative | Total | ||
| Kewei COVID-19 Antigen Rapid Assay | Positive | 143 | 0 | 143 |
| Negative | 14 | 260 | 274 | |
| Total | 157 | 260 | 417 | |
The assay demonstrated acceptable total clinical sensitivity is 91.08% (95% CI: 85.59%-94.61%) when compared to a molecular device produced by Sansure Inc. The assay demonstrated excellent clinical specificity 100% (95% CI: 98.54%-100%). Overall Agreement was 96.64% (94.44%-97.99%).
3. Limit of Detection(LoD)
Limit of detection (LoD) was determined by evaluating different concentrations of heat inactivated SARS-CoV-2 virus. LoD of the SARS-CoV-2 antigen rapid kit was confirmed as 38.5 TCID50/ml.
4. High-dose Hook effect
No high dose hook effect was observed when test with up to a concentration of 6.3×105 TCID50/ml of heat inactive SARS-CoV-2 virus from Academy of Military Sciences PLA China.
| Potential Cross-Reactant | Test Concentration | Result | ||
| Absence of SARS-CoV-2 | presence of SARS-CoV-2 | |||
| Staphylococcus aureus | 1.0×105 TCID50/ml | - | + | |
| Streptococcus pneumoniae | 1.0×106 Cells/ml | - | + | |
| Measles virus | 1.0×105 TCID50/ml | - | + | |
| Mumps virus | 1.0×105 TCID50/ml | - | + | |
| Adenovirus type 3 | 1.0×105 TCID50/ml | - | + | |
| Mycoplasma pneumoniae MP | 1.0×106 Cells/ml | - | + | |
| Parainfluenza type 2 | 1.0×105 TCID50/ml | - | + | |
| Metapneumovirus | 1.0×105 TCID50/ml | - | + | |
| Coronavirus OC43 | 1.0×105 TCID50/ml | - | + | |
| Coronavirus 229E | 1.0×105 TCID50/ml | - | + | |
| Bordetella parapertussis | 1.0×106 Cells/ml | - | + | |
| Influenza B virus (Victoria series) | 1.0×105 TCID50/ml | - | + | |
| Influenza B virus (Y series) | 1.0×105 TCID50/ml | - | + | |
| Influenza A H1N1 (2009) virus | 1.0×105 TCID50/ml | - | + | |
| Influenza A H3N2 virus | 1.0×105 TCID50/ml | - | + | |
| Avian influenza virus H7N9 | 1.0×105 TCID50/ml | - | + | |
| Avian influenza virus H5N1 | 1.0×105 TCID50/ml | - | + | |
| EB virus | 1.0×105 TCID50/ml | - | + | |
| Enterovirus CA16 | 1.0×105 TCID50/ml | - | + | |
| Rhinovirus | 1.0×105 TCID50/ml | - | + | |
![]()
![]()
![]()
![]()