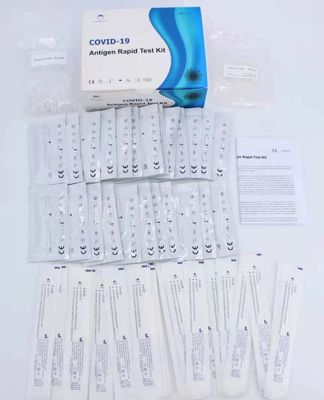
COVID-19 Rapid Diagnostic Throat Test Kits OEM 95.52% Sensitivity
Product Details:
| Place of Origin: | Beijing |
| Certification: | CE; ISO |
Payment & Shipping Terms:
| Minimum Order Quantity: | 10-100pcs |
|---|---|
| Price: | USD1.8-3.5/pc |
| Packaging Details: | 25pcs per case |
| Delivery Time: | 3-5working days |
| Supply Ability: | 1million per week |
|
Detail Information |
|||
| Clinical Specificity: | 100% | Overall Agreement: | 96.21% |
|---|---|---|---|
| Certificate: | CE | Room Temperature Or Refrigerated: | 2-30°C |
| Sensitivity: | 95.52% | Clinical Sensitivity Is 91.08%: | 91.08% |
| Material: | Plastic | ||
| Highlight: | COVID-19 Rapid Diagnostic Throat Test Kits,OEM Rapid Diagnostic Throat Test Kits,OEM Covid-19 Antigen Test Kit |
||
Product Description
ISO Rapid Diagnostic Throast Test Kits Certificated with CE Antigen Test for fresh swabs COVID-19 Antigen China OEM
PERFORMANCE CHARACTERCS
The assay demonstrated excellent clinical specificity 100% (95% CI: 93.98%-100%). Overall Agreement was 97.64% (93.28%-99.19%). The assay demonstrated acceptable total clinical sensitivity is 91.08% (95% CI: 85.59%-94.61%) when compared to a molecular device produced by Sansure Inc. The assay demonstrated excellent clinical specificity 100% (95% CI: 98.54%-100%). Overall Agreement was 96.64% (94.44%-97.99%).
Overall Clinical Performance of Antigen Test Kit
A total of above 157 positive samples and 260 negative samples were detected to evaluate the clinical performance of the COVID-19 Antigen Rapid Test Kit. The Result is below:
| Sansure Covid-19 RT-PCR Assay | ||||
| Positive | Negative | Total | ||
| Kewei COVID-19 Antigen Rapid Assay | Positive | 143 | 0 | 143 |
| Negative | 14 | 260 | 274 | |
| Total | 157 | 260 | 417 | |
The assay demonstrated acceptable total clinical sensitivity is 91.08% (95% CI: 85.59%-94.61%) when compared to a molecular device produced by Sansure Inc. The assay demonstrated excellent clinical specificity 100% (95% CI: 98.54%-100%). Overall Agreement was 96.64% (94.44%-97.99%).
Limit of Detection(LoD)
Limit of detection (LoD) was determined by evaluating different concentrations of heat inactivated SARS-CoV-2 virus. LoD of the SARS-CoV-2 antigen rapid kit was confirmed as 38.5 TCID50/ml.
High-dose Hook effect
No high dose hook effect was observed when test with up to a concentration of 6.3×105 TCID50/ml of heat inactive SARS-CoV-2 virus from Academy of Military Sciences PLA China.
Cross Reactivity
Many types of pneumonia are companied by fever, cough and other respiratory symptoms. In order to eliminate similar clinical symptoms of other types of pneumonia effects, pneumonia mycoplasma, influenza A, pneumococcus etc. were detected for the specific assessment. Each of the organism and viruses were tested in the absence or presence of heat inactivated SARS-CoV-2 virus (308 TCID50/ml), No cross-reactivity or interference was seen.
| Potential Cross-Reactant | Test Concentration | Result | ||
| Absence of SARS-CoV-2 | presence of SARS-CoV-2 | |||
| Staphylococcus aureus | 1.0×105 TCID50/ml | - | + | |
| Streptococcus pneumoniae | 1.0×106 Cells/ml | - | + | |
| Measles virus | 1.0×105 TCID50/ml | - | + | |
| Mumps virus | 1.0×105 TCID50/ml | - | + | |
| Adenovirus type 3 | 1.0×105 TCID50/ml | - | + | |
| Mycoplasma pneumoniae MP | 1.0×106 Cells/ml | - | + | |
| Parainfluenza type 2 | 1.0×105 TCID50/ml | - | + | |
| Metapneumovirus | 1.0×105 TCID50/ml | - | + | |
| Coronavirus OC43 | 1.0×105 TCID50/ml | - | + | |
| Coronavirus 229E | 1.0×105 TCID50/ml | - | + | |
| Bordetella parapertussis | 1.0×106 Cells/ml | - | + | |
| Influenza B virus (Victoria series) | 1.0×105 TCID50/ml | - | + | |
| Influenza B virus (Y series) | 1.0×105 TCID50/ml | - | + | |
| Influenza A H1N1 (2009) virus | 1.0×105 TCID50/ml | - | + | |
| Influenza A H3N2 virus | 1.0×105 TCID50/ml | - | + | |
| Avian influenza virus H7N9 | 1.0×105 TCID50/ml | - | + | |
| Avian influenza virus H5N1 | 1.0×105 TCID50/ml | - | + | |
| EB virus | 1.0×105 TCID50/ml | - | + | |
| Enterovirus CA16 | 1.0×105 TCID50/ml | - | + | |
| Rhinovirus | 1.0×105 TCID50/ml | - | + | |
![]()
![]()
![]()